
What would be the best way to automatically adjust BC for a group of images to some level that I set, like a reference image? Normalize histogram and brightness usually goes way to far and isn't helpful. The BC on each image sometimes differs and it is up to me to manually adjust it so they all look the same and I can run a batch analysis on them. I'm just measuring the black interiors.Īnother, much simpler, issue is image consistency. This helps but some of the smaller less defined particles are still difficult to see. To get rid of the white halo around the particles (or pits rather) I perform a grey morphology filter and adjust the BC. There are so many options in Weka I'm not even sure which would best suit my analysis. Currently I am using Weka Trainable Image Segmentation (in Fiji) for this and it's given me the best results but it still struggles. I need to segment them so that I can count the particles and analyze their shape, distribution, aspect ratio, etc automatically. This is what my images tend to look like. One of the biggest issues I'm encountering is consistent particle counting for my images. I'm currently working through a project that involves a fair bit of image capture, processing, and analysis. ¹This subreddit is not affiliated with the creators of ImageJ or FIJI, but is simply a place to share ideas, papers, resources, and expertise - especially as relate to questions & answers posted here. Sign-up for one of the mailing lists: /Mailing_Lists It also hosts a forum for interacting with the developers.įIJI Is Just ImageJ - "a distribution of ImageJ (and ImageJ2) together with Java, Java3D and a lot of plugins organized into a coherent menu structure."
#IMAGEJ PARTICLE ANALYSIS FULL#
is full of ImageJ development and analysis resources. Image analysis is interdisciplinary, so clearly explain field-specific terms or jargon. Clearly explain what you are trying to learn, not just the method used, to avoid the XY problem. Provide details: Be thorough in outlining the question(s) that you are trying to answer.People from the future may be stuck trying to answer the same question. Report spam or content that is hateful or off-topic.Upvote those who contribute to the discussion and provide freely of their time to assist you.Projects: Share a Link to your pet image analysis project.Research: Links to published (articles in scientific journals or in established repositories) that utilize ImageJ/FIJI for image analysis or are about image analysis.Discussions: Text posts, meant to ask about general issues relating to image analysis.Image analyst job posts are also welcome. Tips: Text or Link posts to share useful how-to tricks and discoveries on using ImageJ/FIJI.Questions which have been Solved will be marked as such. This could include algorithms, microscopy and scientific imaging, plug-ins, methods, and specific features of the software. Questions: Text posts asking about image analysis and ImageJ/FIJI.
#IMAGEJ PARTICLE ANALYSIS SOFTWARE#
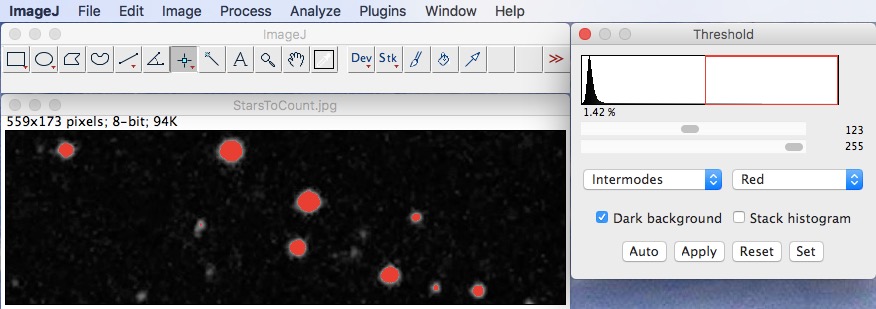
This is a an unofficial¹ forum to discuss image analysis, software features, to get help, to share ideas, and to share work done using ImageJ or FIJI. It's used worldwide, by a broad range of scientists. Adjust the Threshold for the image.ImageJ is a freely available, open source image processing and analysis program using Java, on which FIJI is based.Transfer images to 8- or 16-bit format: Image/ Type/ 16-Bit.Repeat lines measurements as many times as required.Ctrl-T to put the measurements in ROI Manager.Measure smallest and biggest cells to be included in counting.Save the image for your reference (File/ Save as/ Tiff).Click on Results button to see the results of counting on this image.Use different colors at the bottom of the "Counter Window" to mark cells of interest.Plugins/ Particles Analysis/ Cell Counter to count up to 4 different cell types.Plugins/ Particles Analysis/ Grid to put a grid to the image.Define number of points of grids in your image (9x9 = 81).Calibrate the image to make measurements in micrometers.


 0 kommentar(er)
0 kommentar(er)
